Abnormal pulmonary neuroendocrine cells (PNECs) are found in various conditions including carcinoid tumors, diffuse idiopathic neuroendocrine cell hyperplasia (DIPNECH), large cell neuroendocrine carcinoma, and small cell lung cancer. Additionally, PNECs are increased and abnormally distributed in pediatric respiratory disorders such as neuroendocrine cell hyperplasia of infancy (NEHI), where infants exhibit hypoxemia, tachypnea, and failure to thrive.
In both NEHI and DIPNECH, patients exhibit prominent obstructive ventilatory physiology. These disorders, characterized by primary neuroendocrine cell expansion, provide a unique insight into the diverse physiology of PNECs. We are systematically investigating these with single-cell precision in human lung tissues.
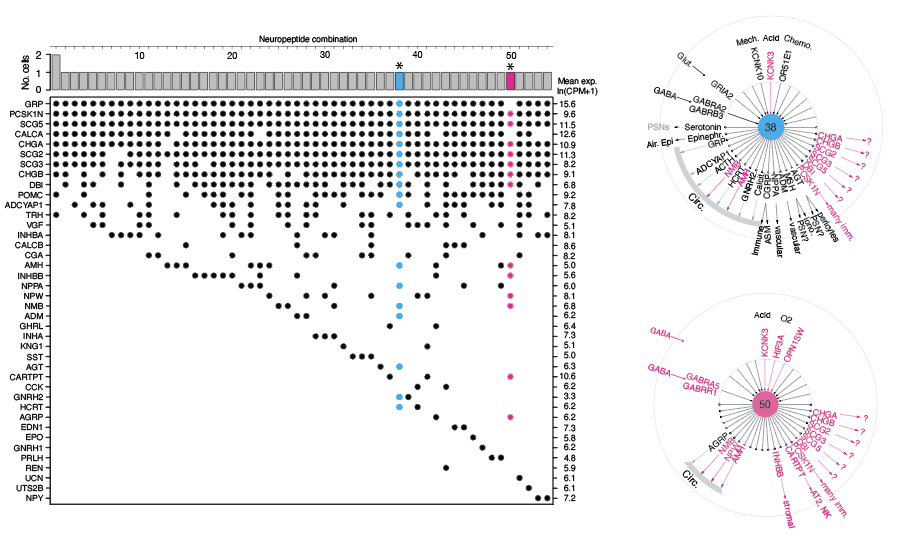
Our research involves determining the molecular profiles of individual cells in neuroendocrine diseases to understand the bioactive signals released from PNECs, their targets, and cellular functions. For example, we highlight the incredible diversity of normal human NE cells and the diverse signals present in a human carcinoid by single-cell RNA-sequencing (Kuo et al., Fig. 6).
We study individual carcinoid patients with single-cell precision in human tissues. Our human studies are interdisciplinary and collaborative, involving a wide range of scientific expertise.
Pulmonary Neuroendocrine (NE) Cell Development
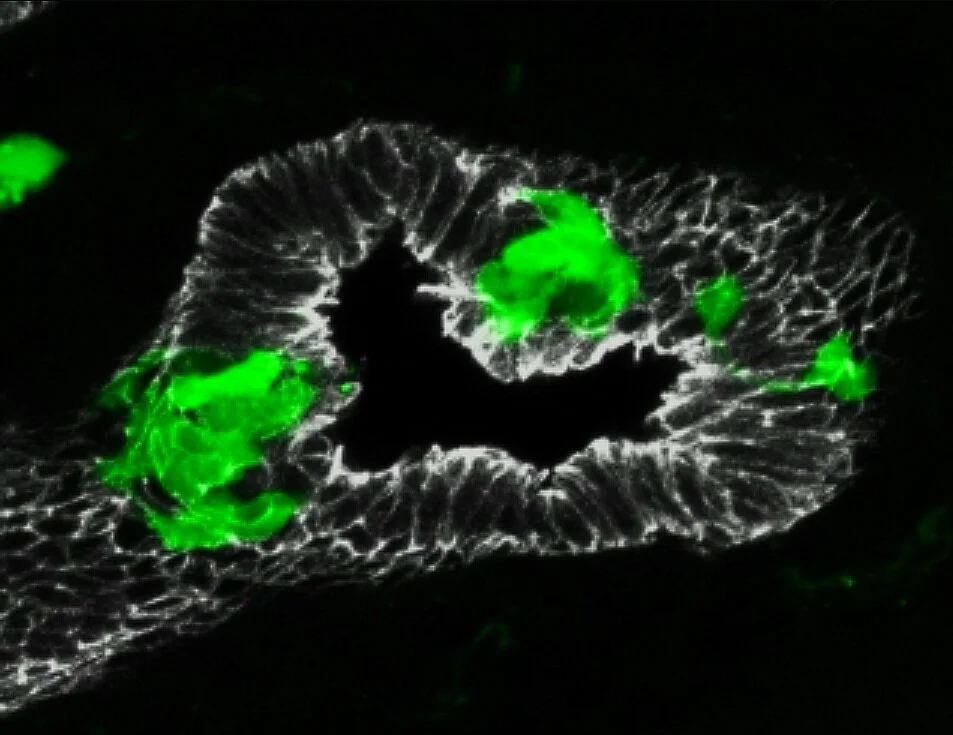
Pulmonary neuroendocrine cells (PNECs) are most prominent during fetal and early postnatal lung development. They express a variety of neuropeptides, neurotransmitters, and hormones, including gastrin-releasing peptide, calcitonin, and calcitonin gene-related peptide. We recently identified the remarkable diversity of NE signals (Kuo et al, 2022). PNECs cluster at airway branchpoints to form structures called neuroepithelial bodies (NEBs), which are uniquely organized, innervated within the airway epithelium.
An abnormally increased number of NEBs is associated with several infant respiratory disorders, such as neuroendocrine cell hyperplasia of infancy (NEHI), sudden infant death syndrome (SIDS), and bronchopulmonary dysplasia (BPD). These disorders disrupt normal development, leading to symptoms like respiratory distress, low blood oxygen levels, and failure to thrive in infants with NEHI.
To investigate the onset of these diseases linked to improper neuroendocrine cell development, we study how these clusters form, their typical locations in humans, and the signals they emit. Our research employs in vivo genetic cell labeling, high-resolution live imaging, and single-cell transcriptomics, using both animal models and human tissue samples.
PNECs in human lung diseases
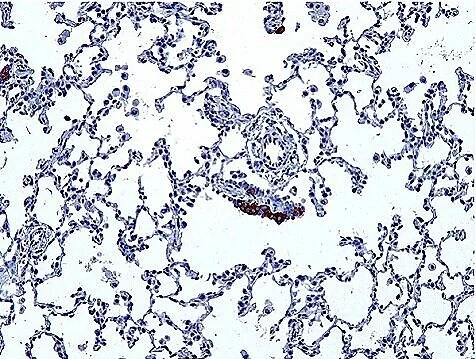
Abnormal PNECs in a NEHI patient
Diversity of NE cell signals
Molecular interrogation and understanding of neuroendocrine (NE) cell diversity and function in the lung has been historically limited by their scarcity; they constitute approximately 0.01% of human lung cells. To address this challenge, we employed single-cell RNA sequencing to isolate and profile hundreds of pulmonary neuroendocrine cells, initially in mice and later extending to human lung tissue.
Our systemic and comprehensive analysis of the expressed neuropeptide and hormone genes revealed over 30 expressed genes. Further analysis of each individual neuroendocrine cell highlighted the diversity of sensory ion channels and neurotransmitter receptors individual PNECs expressed.
Schematics above show examples of single-cell profiles from human NE cells, which expressed a greater number of peptidergic hormone genes compared to those from mice.